1. Advanced Energy Materials: Fe2O3 Nanoparticle Catalyst Enhances Recyclability of Deep Charge and Discharge Performance of Aprotic Li-O2 Batteries

Fig.1 Schematic diagram of the mechanism of "epitaxial induced nucleation and growth" of Li2O2 proposed on Fe2O3
While the high energy density of Li2O2 chemistry is promising for commercial applications of electric vehicles, the instability of carbon-based cathodes and the insulation of discharge products limit their rechargeability and energy density. Recently, Professor Marnix Wagemaker (Corresponding author) of Delft University of Technology in the Netherlands proposed a cathode material composed of α-Fe2O3 nanoparticles and carbon nanotubes (CNT), which is excellent in high-capacity deep charge and discharge. Cyclic stability. The initial capacity of the Fe2O3/CNT electrode reached 805 mAhg-1 (0.7 mA h cm-2) at 0.2 m A cm-2 while maintaining a capacity of 1098 mAhg -1 (0.95 mA h cm-2) after 50 cycles. On-site structure, spectroscopy and morphological analysis of the evolution of Li2O2 indicate that Li2O2 preferentially grows on Fe2O3. The (104) interplanar spacing of the (100) and Fe2O3 planes similar to Li2O2 indicates that the latter epitaxially induces Li2O2 nucleation. This results in larger Li2O2 major grains and smaller secondary particles, which enhances the reversibility of Li2O2 formation and results in a more stable interface within the electrode compared to secondary particles deposited on CNTs. This bifunctional material acts as both a stable bulk substrate and a redox reaction in Li2O2 cells, providing new opportunities to optimize the morphology of the discharge product for high cycle stability and coulombic efficiency.
2. Advanced Materials: Stability of high performance Pt-based catalysts for oxygen reduction

Fig. 2 Schematic diagram of the influence of surface morphology and electronic surface state on ORR kinetics
Due to environmental sustainability and high efficiency, proton exchange membrane fuel cells (PEMFCs) are expected to become important energy types for electric vehicles, energy production and the aerospace industry in the coming decades. Recently, Prof. Yu Zhuoping from Tongji University (corresponding author) reviewed the latest developments in shape-controlled nanostructured catalysts, focusing on the stability of high performance platinum-based catalysts and related mechanisms. Due to stability issues, especially in membrane electrode assemblies (MEAs), catalysts with high activity are far from meeting the requirements of their applications. Therefore, a method for solving the overall performance of a Pt-based catalyst is discussed herein.
3. J. Am. Chem. Soc.: Monoatomic Au/NiFe Layered Double Hydroxide Electrocatalysts - Exploring the Origin of Oxygen Reduction Activity

Fig. 3 Schematic diagram of a two-layer plate model with sAu/NiFe LDH with interlayer CO32- and water molecules
A basic understanding of the origin of oxygen evolution reaction (OER) activity of transition metal electrocatalysts, especially the single noble metal atoms supported on layered double hydroxides (LDHs), which are useful for further energy conversion techniques for designing highly efficient electrocatalysts. Help. Recently, Professor Zhang Bing of Tianjin University and Professor Wang Wechao (co-author) of Nankai University proposed to support the monoatomic Au on NiFe LDH (sAu/NiFe LDH) to clarify the active source of LDHs system and enhance the OER activity of 0.4wt% sAu modification. 6 times. Combined with theoretical calculations, the activity of NiFe LDH is derived from the in-situ NiFe oxyhydroxide of LDH during OER. With the advent of sAu, sAu/NiFe LDH has an overpotential of 0.21 V compared to the calculated result (0.18 V). The team believes that the excellent OER activity of sAu/NiFe LDH is attributed to the formation of stable NiFe oxyhydroxide at the interface between CO32- and H2O and LDH, which is adjacent to sAu and redistributes the charge of active Fe and its surrounding atoms.
4. Science Advances: In situ formation of molecular Ni-Fe active sites on heteroatom-doped graphenes for heterogeneous electrocatalysts for oxygen evolution

Figure 4 DFT calculation derivative model for indicating the binding of Ni(acac)2 to HG
At the hetero interface, the Ni site is derived from Ni2+ doped into heteroatom-doped graphene. The molecular Ni site on graphene has redox activity. However, they exhibit an activity of poor oxygen evolution reaction (OER) in an aqueous KOH solution. Recently, Professor Wang Wei (communication author) of Nanyang Technological University in Singapore demonstrated for the first time that the presence of Fe3+ in solution can be bonded at a Ni site at a distance of 2.7A, resulting in molecular size and heterogeneous Ni fixed on doped graphene. -Fe site. These Ni-Fe sites showed significantly improved OER activity. The formation of the Ni-Fe site was confirmed in the picogram (potential-pH diagram), and it was revealed that the Ni-Fe site adsorbed the HO-ion having a bridge geometry, which promoted OER electrocatalysis.
5. Science Advances: Selective Electrochemical Nitrogen Immobilization Using Reticulated Chemistry

Figure 5 Schematic diagram of Ag-Au@ZIF as a water repellent and N2 molecular concentrate for electrochemical NRR
Electrochemical nitrogen-ammonia immobilization as a sustainable strategy can solve energy-intensive operations through the process of ammonia production by Haber-Bosch. However, current advances in electrochemical nitrogen reduction (NRR) have hampered the overwhelming competition for hydrogen evolution reactions (HER) and the high temperature/high pressure requirements of all conventional NRR catalysts. Recently, Professor Xing Yi Ling (Corresponding author) of Nanyang Technological University, Singapore, achieved excellent NRR selectivity (?90%) and improved by coating a superhydrophobic metal-organic framework (MOF) layer on an NRR electrocatalyst. 10% Faraday efficiency. The reticular chemistry method uses the hydrophobic and molecular concentration effects of MOF to overcome the bottleneck of strong HER, revealing the unprecedented electrochemical characteristics of NRR, which is crucial for future theoretical research.
6. Science Advances: Significant progress in achieving high power and stable non-precious metal catalyst MEA for proton exchange membrane fuel cells

Figure 6 Polarization analysis of MEA prepared from three different catalysts under air and oxygen
Despite significant advances in the development of non-precious metal catalysts (NPMCs) over the past few decades, the performance and stability of these promising catalysts have not yet reached the commercial level of proton exchange membrane fuel cells (PEMFCs). Recently, Ballin Power Systems' Dustin Banham and Siyu Ye (co-communication author) confirmed the highest performance of the NPMC-based membrane electrode assembly (MEA) through the rational design of the cathode catalyst layer (CCL), achieving 570mW in air. The peak power of cm2. This record-breaking performance is achieved by using almost all pre-commercial catalysts with pore sizes less than 3 nm, which challenges previous beliefs that larger catalyst pores are needed to achieve high current densities. In-situ electrochemical analysis of CCL is also used to help gain insight into the degradation mechanisms observed during constant current testing. Overall, the performance of this NPMC-based MEA has reached commercial readiness and will be available for portable power applications.
7. Advanced Materials: Stability of high performance Pt-based catalysts for oxygen reduction

Figure 7 (a) Schematic diagram of a microreactor with a HER three-electrode device. (b) Schematic cross-section of the electrochemical device
For the electrochemical hydrogen evolution reaction (HER), the electrical properties of the catalyst can play an important role in affecting the overall catalytic activity. This is particularly important for semiconductor HER catalysts such as MoS2, which has been extensively studied over the past decade. Recently, Professor Judy J. Cha (communication author) of Yale University in the United States used the on-chip microreactors on two model catalysts (semiconductor MoS2 and semi-metal WTe2) to derive the influence of individual factors and study them with HER catalysis. The relationship of activity. The results indicate that electron injection at the catalyst/collector interface and intra- and inter-layer charge transfer of the catalyst are more important than thermodynamic energy considerations. For WTe2, since the microreactor allows for accurate measurement of the type and area of ​​the catalytic site, the relationship between pure site activity and pure thermodynamics and overall activity is measured and established. This method provides an opportunity to systematically study electrochemical reactions to help establish rational design principles for future electrocatalysts.
8. Energy & Enviro nmental Science: Hybrid acid functionalized fluoroelastomer with excellent chemical durability and vehicle fuel cell performance

Figure 8: Total synthesis reaction scheme for synthesis of PolyHPA (final product) from FC-2178 and DHPP
In order to further promote the commercialization of automotive fuel cells, it is necessary to solve the durability problem. The addition of mechanical supports to the membrane currently adequately addresses the problem of mechanical degradation, but the attack by chemical degradation of oxygenated free radicals remains an unsolved challenge. Typical mitigation strategies use ruthenium or manganese species as free radical scavengers, but these ions can migrate in the membrane and even leach out of the system. Recently, Professor Andrew M. Herring (Corresponding author) of the University of Colorado School of Mines, USA, immobilized 11-tungstic acid (HSiW11) (a defective Keggin structure of HPA) with a fluoroelastomer as a free radical decomposition catalyst and Proton conducting acid. This dual function allows for high levels of free radical scavenging material and high ion exchange capacity. This material exhibits a significant increase in proton conductivity above 60 ° C due to softening of the polymer. PolyHPAs have outstanding chemical stability and very low in situ high frequency resistance (HFR) compared to Nafion® N211 fuel cells during fuel cell testing. Under the load of 75 wt% HSiW11, the HFR of the fuel cell is 22% lower than that of N211.
9. Physical Review Letters: Catalytic Active Surface Phase Identification of PtRh Nanoparticles Catalyzed by CO Oxidation under Catalytic Reaction Conditions

Fig. 9 Orientation and size characterization of Pt-Rh alloy particles
Pt-Rh alloy nanoparticles on oxide supports are widely used for heterogeneous catalysis, ranging from automotive exhaust control to energy conversion. In order to improve catalyst performance, the atomic scale correlation of the surface structure of the nanoparticles with their catalytic activity is critical under industrial operating conditions. Recently, Prof. A. Stierle and Prof. U. Hejral (co-communication author) of the German Institute of Electronic Synchrotrons proposed X-ray diffraction data sensitive to the surface structure of nanoparticles in combination with in situ mass spectrometry in CO oxidation process close to ambient pressure. We identify the formation of ultra-thin surface oxides by detecting X-ray diffraction signals from specific nanoparticles and correlate their evolution to the enhanced catalytic activity of the sample. The team's approach opens the door to an in-depth characterization of an oxide-assisted nanoparticle catalyst with unprecedented atomic-scale resolution.
10. ACS Nano: Study on Mechanism of Photocatalytic Hydrogen Desorption from Palladium Surface by Partial Surface Plasmon Resonance

Fig. 10 Schematic diagram of HMARCs in realizing H2 light absorption on Pd surface in embedded correlation wave function (ECW) theory
Nanoparticles synthesized from plasmonic metal can absorb low energy light, producing oscillations/excitation of available valence electron density. For example, heterogeneous photocatalysis can be achieved in a heterometallic antenna-heteroplex (HMARC) by coupling an active agent that undergoes a chemical reaction to the plasmonic nanoparticles. Recently, Professor Emily A. Carter (Corresponding author) of Princeton University in the United States explored the rate limiting step of hydrogen absorption from the surface of Pd in ​​the theoretical framework to understand the mechanism of photodesorption in the HMARC module. It is essential to realize acetylene. Partial rather than fully hydrogenated. In order to properly describe the electronic excited state of the metal-molecular system, the team adopted the embedded fully active space self-consistent field and the n-electron valence state perturbation theory in the density functional embedding theory. The results of these calculations indicate that the light absorption mechanism does not produce frequently induced transient anion species, but rather increases the available excited state, a negligible low-barrier pathway for charge transfer characteristics.
Toggle switches
Toggle switches, also called On Off Toggle Switches, is often used as the switching device of the equipment stalls. Meanwhile, we are also offer our customers Key Switches, Metal Switches, Automotive Switches, Push Button Switches, etc.
The Electrical Toggle Switches is a manually controlled Toggle Switches similar to the dial switch. Most of this Latching Toggle Switches are widely used in on-off control of AC and DC power circuits, and are less commonly used in circuits of several kilohertz or up to 1 megahertz. Let's take a look at the following.
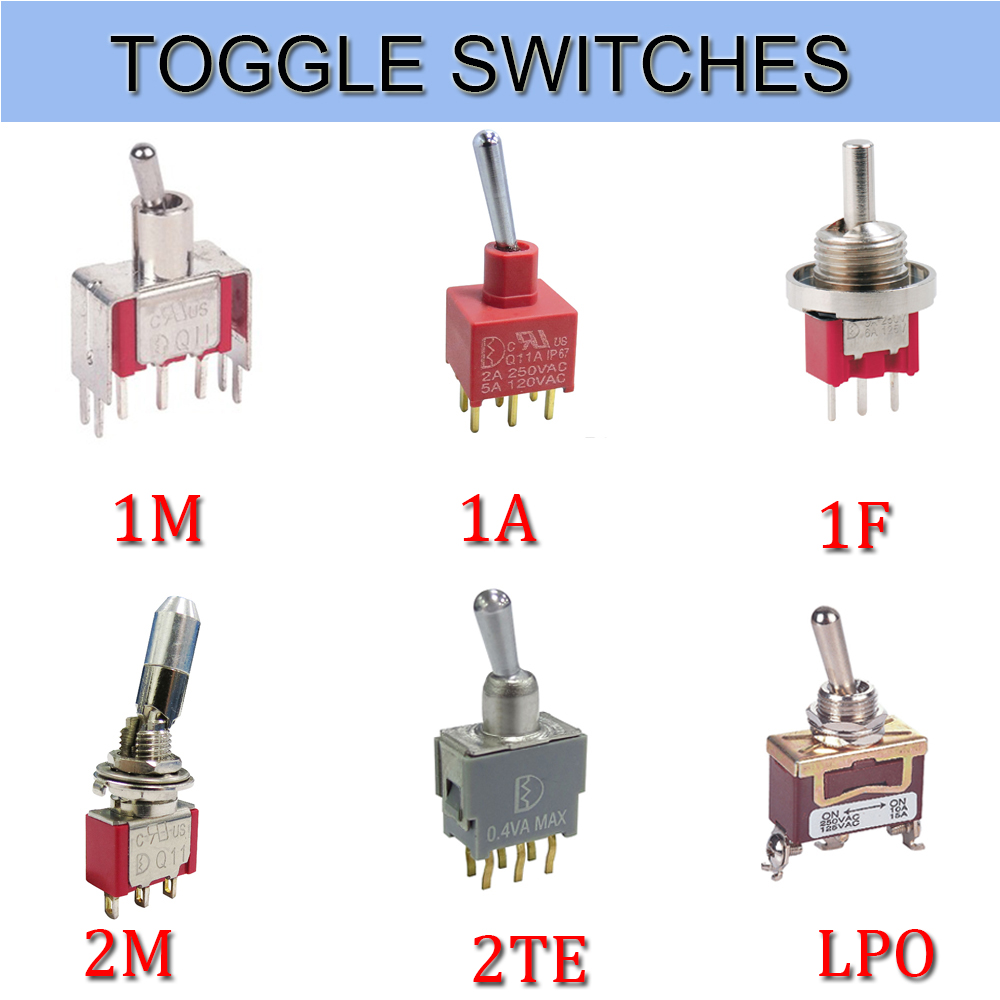
1. Splash-proof knob button switch
The panel is installed with a splash-proof `O` ring seal, and the knob is a ball. It is a splash-proof ball button knob switch. Its terminals are in a straight line and the bottom of the terminals is sealed with epoxy resin. Strong corrosion resistance, suitable for automotive parts
2. Vertical Mount Right Angle Toggle Switch
The vertical mounting of the terminals and the terminal pins are right-angled, so it is a vertically mounted right-angled toggle switch. Its contacts are gold-plated and highly reliable. Mostly used in anti-theft devices, alert system.
3. Bipolar single toggle switch
At the same time, the switch breaks the phase line and the N line and controls one branch. Therefore, it is a bipolar single toggle switches. The contacts are in 3PDT form and are used for multimedia speakers and stereos.
4. Standard surface mount unthreaded toggle switches
The terminal adopts the standard mounting mode. Its sleeve has no thread. It is called a standard surface mount screw-less switch. The contacts are SPDT and its electrical life is as high as 55,000. Used for medical equipment
5. Horizontally mounted right-angle toggle switch
Compared to the vertical switch, it only changes direction to horizontal, so it is horizontally mounted right-angle toggle switch. The contacts are double-pole double-throw and the bottom of the terminal is Epoxy Seal. Mostly used for computer peripherals.

Toggle Switches,Toggle Switch On Off,Toggle Switch Autozone,Toggle Switch Home Depot
YESWITCH ELECTRONICS CO., LTD. , https://www.yeswitches.com